Are you seeking for 'how to write a complete ionic equation'? You can find all of the material on this webpage.
Table of contents
- How to write a complete ionic equation in 2021
- Practice problems on net ionic equations key
- Net ionic equation worksheet
- Net ionic equation vs complete ionic equation
- How to write ionic formula
- What is a complete ionic equation
- Ionic equation of nacl
- What is a complete ionic equation apex
How to write a complete ionic equation in 2021
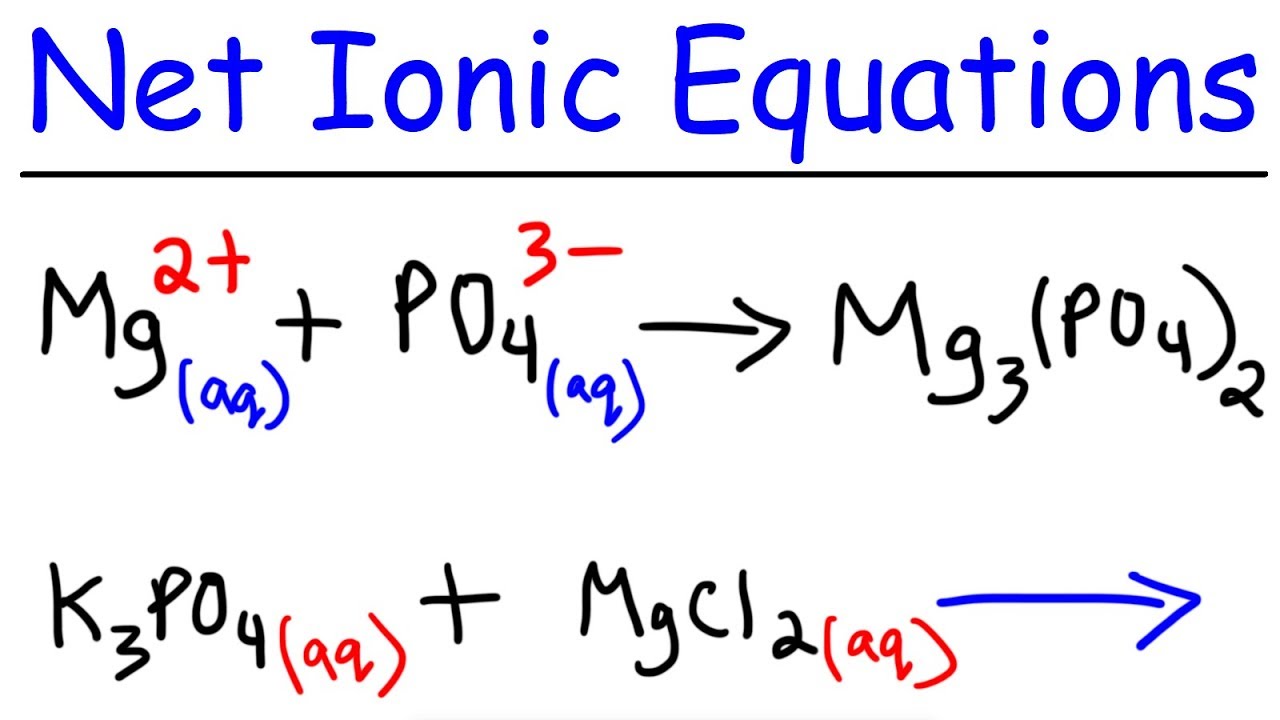 This picture demonstrates how to write a complete ionic equation.
This picture demonstrates how to write a complete ionic equation.
Practice problems on net ionic equations key
 This image demonstrates Practice problems on net ionic equations key.
This image demonstrates Practice problems on net ionic equations key.
Net ionic equation worksheet
 This image illustrates Net ionic equation worksheet.
This image illustrates Net ionic equation worksheet.
Net ionic equation vs complete ionic equation
 This picture illustrates Net ionic equation vs complete ionic equation.
This picture illustrates Net ionic equation vs complete ionic equation.
How to write ionic formula
 This image representes How to write ionic formula.
This image representes How to write ionic formula.
What is a complete ionic equation
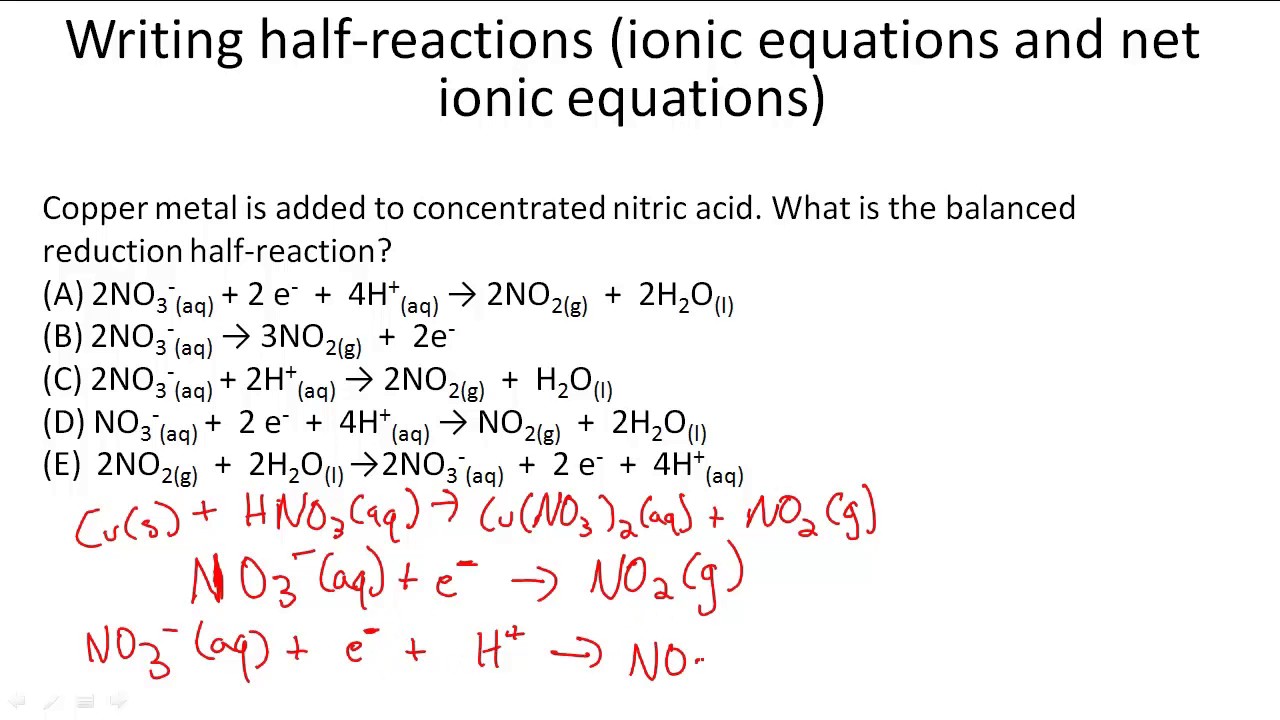 This image shows What is a complete ionic equation.
This image shows What is a complete ionic equation.
Ionic equation of nacl
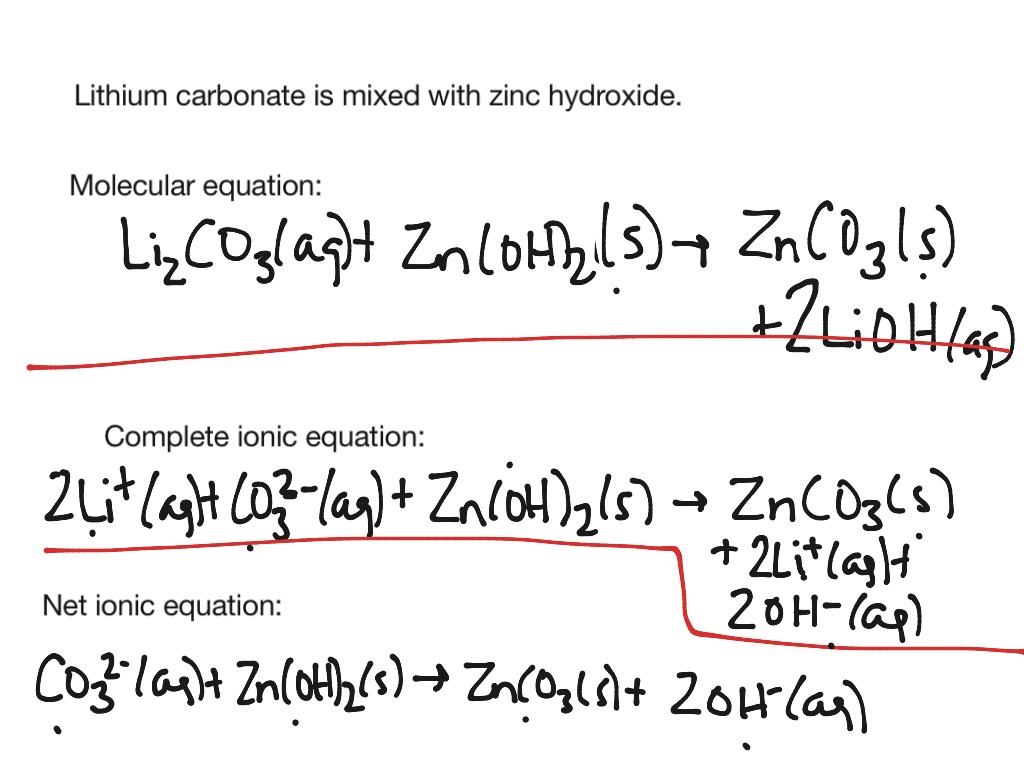 This picture illustrates Ionic equation of nacl.
This picture illustrates Ionic equation of nacl.
What is a complete ionic equation apex
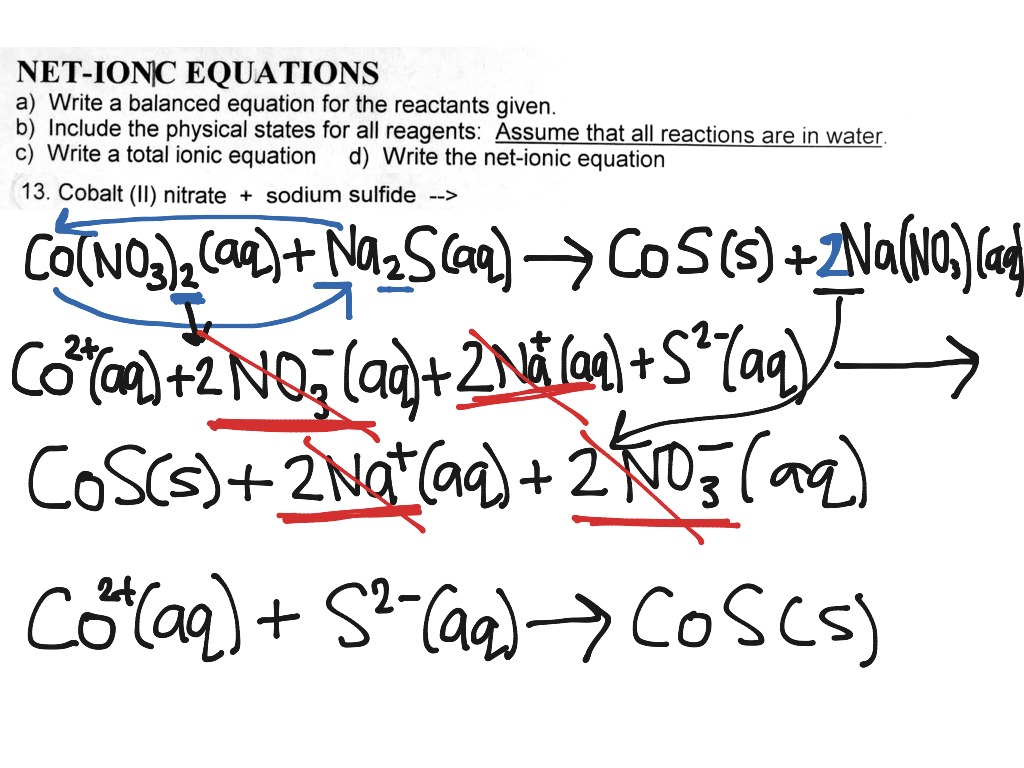 This image representes What is a complete ionic equation apex.
This image representes What is a complete ionic equation apex.
How to write an ionic equation in chemistry?
(Spectator ions are ions that do not take part in the reaction) Reaction between sodium hydroxide and sulphuric acid to form sodium sulphate and water. Change the chemical equation to ionic equation: Na+ +OH− + H+ +SO2− 4 → Na+ + SO2− 4 + H2O Na + + OH − + H + + SO 4 2 − → Na + + SO 4 2 − + H 2 O
How to write an ionic equation for neutralisation?
Ionic Equations for Neutralisation STEP 1: Write the chemical equation HCl (aq) + NaOH (aq) ⟶ NaCl (aq) + H 2 O (l) STEP 2: Rewrite by separating the soluble ionic compounds into their dissociated ions H + (aq) + Cl – (aq) + Na (aq) +... STEP 3: Cancel out common ions, which are the spectator ions H ...
How is a net ionic equation different from a complete ionic equation?
That means that sometimes we might have to divide all of the stoichiometric coefficients by a common divisor as the last step to get the final form of the net ionic equation. A net ionic equation shows only the chemical species that are involved in a reaction, while a complete ionic equation also includes the spectator ions.
How to write the ionic equation for sulfuric acid?
Write the ionic equation for the acid-metal reaction between zinc and sulfuric acid to form zinc sulfate salt and hydrogen gas. Chemical equation: Zn(s) + H 2 SO 4 (aq) ZnSO 4 (aq) + H 2 (g) Ionic equation: Zn(s) + 2H + (aq) Zn 2+ (aq) + H 2 (g)
Last Update: Oct 2021